For End Users
A Better Overall Support Experience
As the manufacturer, we can offer vastly superior troubleshooting and RMA (Return Merchandise Authorization) experience compared to a rebrander. If your Cybernet computer or tablet fails, we have engineering-trained technicians who can begin troubleshooting the issue right over the phone or through email. If the issue requires further troubleshooting, the device can be sent directly to the nearest Cybernet location in your region and is typically worked on within twenty-four hours after the unit's arrival.
If a device sold by a rebrander fails, the customer will first send it to that rebrander for repairs. However, the rebrander won't have the technical skills, equipment, or spare parts to fix the device.
Instead, they'll have to send it to the actual manufacturer of that device, which might be hundreds of miles away or even overseas, drastically increasing the turnaround time. Even then, once the device arrives back at the original manufacturer, there's no guarantee that they keep spare parts in stock. Most companies in the tech sector rely on just-in-time inventory rather than stocking an inventory of spares. There's a good chance that there simply won't be any spare parts available until the next production run, which could be weeks or months away. You need to ask yourself: Does your hospital or practice want to wait potentially months for one of its computers to get repaired?
Access to Assembly and Testing Documentation
Another reason for choosing a true ODM such as Cybernet is that if one of our devices fails, we have full access to that device's history. We can check its operators, initial quality check, burn-in test log, and more to determine if there were any discrepancies to previous builds or orders. Rebranders simply do not have access to this kind of data.
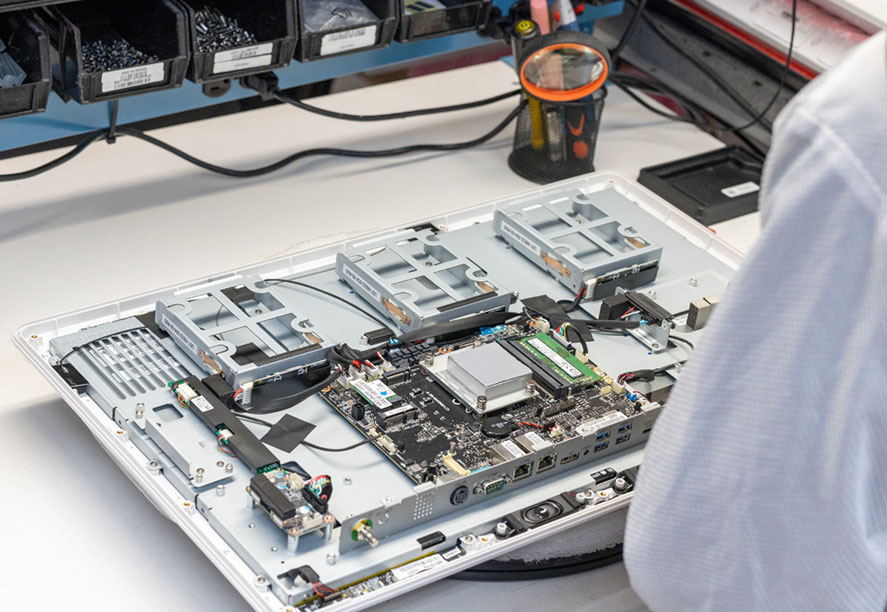
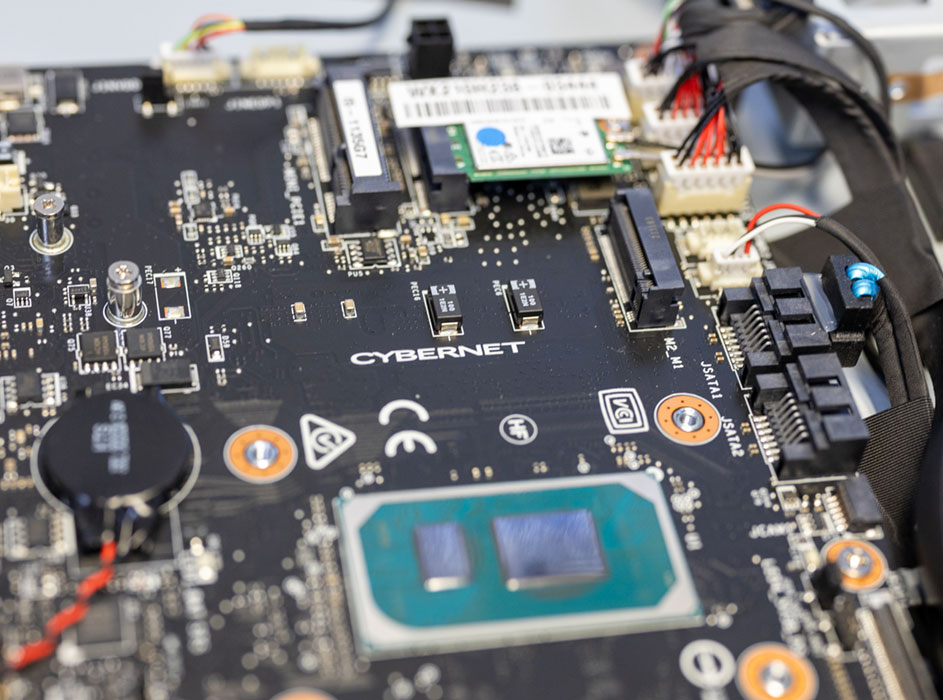
These are just a few ways we take our responsibilities as a true manufacturer seriously. We also do everything in our power to ensure our computers and tablets don't fail in the first place. Every device we make goes through an exhaustive assembly, testing, and documentation process, with every component placed precisely as part of our QMS ISO: 13485 medical certification.
Rigorous Testing and Lower Failure Rates
We also conduct a 12-hour burn-in test for every single device we sell. During these twelve hours, the device is pushed to the limits of its processing power and memory to ensure that everything in the device is functioning properly and that it won't fail under operating stress. To be clear, we don't mean a single sample from an order or production run but every single computer or tablet that leaves our assembly line. That's why our products boast a failure rate of under 0.5% over a 7-year life expectancy.